China Localized Drug Delivery for Cancer Hospitals
China Localized Drug Delivery for Cancer HospitalsThis article provides a comprehensive overview of localized drug delivery systems for cancer treatment within Chinese hospitals, focusing on current advancements, challenges, and future prospects. It examines various delivery methods, regulatory considerations, and the crucial role of research and development in improving patient outcomes.
China Localized Drug Delivery for Cancer Hospitals: Advancing Cancer Treatment
The fight against cancer in China demands innovative approaches to treatment. China Localized Drug Delivery for Cancer Hospitals is a rapidly evolving field, offering significant potential to improve patient outcomes and quality of life. This article explores the key aspects of localized drug delivery systems, their application within the Chinese healthcare landscape, and the challenges that need to be addressed for wider adoption.
Types of Localized Drug Delivery Systems
Targeted Nanoparticles
Nanoparticle-based drug delivery systems offer precise targeting of cancerous cells, minimizing systemic toxicity. These nanoparticles, often functionalized with targeting ligands, can deliver chemotherapeutic agents, immunotherapy drugs, or other therapeutic agents directly to the tumor site. Research in this area is actively ongoing in China, with several institutions exploring novel nanoparticle designs and formulations. The Shandong Baofa Cancer Research Institute (https://www.baofahospital.com/) is at the forefront of this research.
Targeted Liposomes
Liposomes are another promising approach to China Localized Drug Delivery for Cancer Hospitals. These spherical vesicles encapsulate therapeutic agents and can be engineered to target specific tumor cells. Their biocompatibility and ability to enhance drug solubility make them an attractive option. Studies have shown their efficacy in various cancer types, and ongoing clinical trials in China are further evaluating their potential.
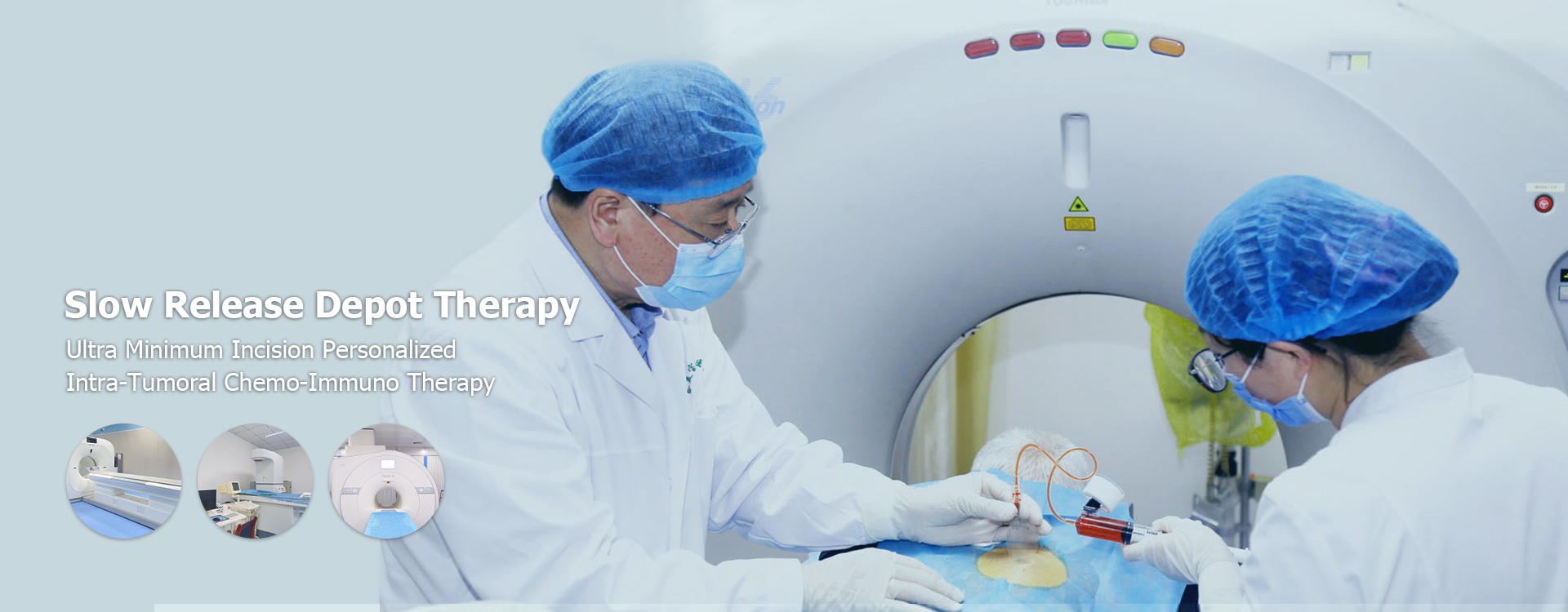
Implantable Drug Reservoirs
Implantable drug delivery systems offer sustained release of therapeutic agents directly at the tumor site. This approach can improve therapeutic efficacy while reducing the frequency of systemic administrations. Research and development in implantable devices specifically designed for the Chinese population's unique needs are crucial for optimizing their benefits.
Regulatory Landscape and Challenges
The regulatory environment surrounding China Localized Drug Delivery for Cancer Hospitals is constantly evolving. The China Food and Drug Administration (CFDA) plays a vital role in ensuring the safety and efficacy of new drug delivery systems. Navigating the regulatory pathway requires careful planning and compliance with stringent guidelines. Challenges include the need for robust clinical trials, demonstrating efficacy and safety in the Chinese population, and addressing potential manufacturing complexities.
Future Directions and Research
The future of China Localized Drug Delivery for Cancer Hospitals is bright, with significant potential for advancements in personalized medicine. Further research is needed to develop more efficient and targeted delivery systems, address drug resistance, and minimize side effects. Collaborative efforts between researchers, pharmaceutical companies, and regulatory bodies are essential to accelerate innovation and make these advanced therapies accessible to cancer patients in China.
Comparative Analysis of Different Drug Delivery Systems
| Drug Delivery System | Advantages | Disadvantages |
|---|---|---|
| Targeted Nanoparticles | High target specificity, reduced systemic toxicity | Production challenges, potential immune response |
| Targeted Liposomes | Biocompatible, enhanced drug solubility | Limited payload capacity, potential for leakage |
| Implantable Drug Reservoirs | Sustained drug release, reduced administration frequency | Surgical procedure required, potential for complications |
Further research is crucial to overcome these challenges and unlock the full potential of localized drug delivery for cancer treatment in China.
Disclaimer: This information is for educational purposes only and should not be considered medical advice. Always consult with a healthcare professional before making any decisions related to your health or treatment.
Related products
Related products
Best selling products
Best selling products-
 Mark, a prostate cancer bone metastasis patient from the United States
Mark, a prostate cancer bone metastasis patient from the United States -
 Anthony, lymphocytic cancer patient from the United States 24
Anthony, lymphocytic cancer patient from the United States 24 -
 PAT, rectal cancer patient from the United States
PAT, rectal cancer patient from the United States -
 Andress, a 9-year-old boy from the United States
Andress, a 9-year-old boy from the United States -
 Famous American female painter Muriel
Famous American female painter Muriel -
 Nell Smith, a throat cancer patient from Switzerland
Nell Smith, a throat cancer patient from Switzerland
Related search
Related search- Cheap stage 3b lung cancer treatment Hospitals
- best hospital for lung cancer treatment cost
- Cheap cancer care hospital Hospitals
- China pancreatitis symptoms Hospitals
- treatment lung cancer treatment medications
- treatment breast cancer symptoms Hospitals
- kidney cancer causes
- stage 4 renal cell carcinoma
- Cheap new prostate cancer treatment liquid radiation near me
- treatment secondary lung cancer treatment near me