- English
- Chinese
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Irish
- Greek
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
- Kinyarwanda
- Tatar
- Oriya
- Turkmen
- Uyghur

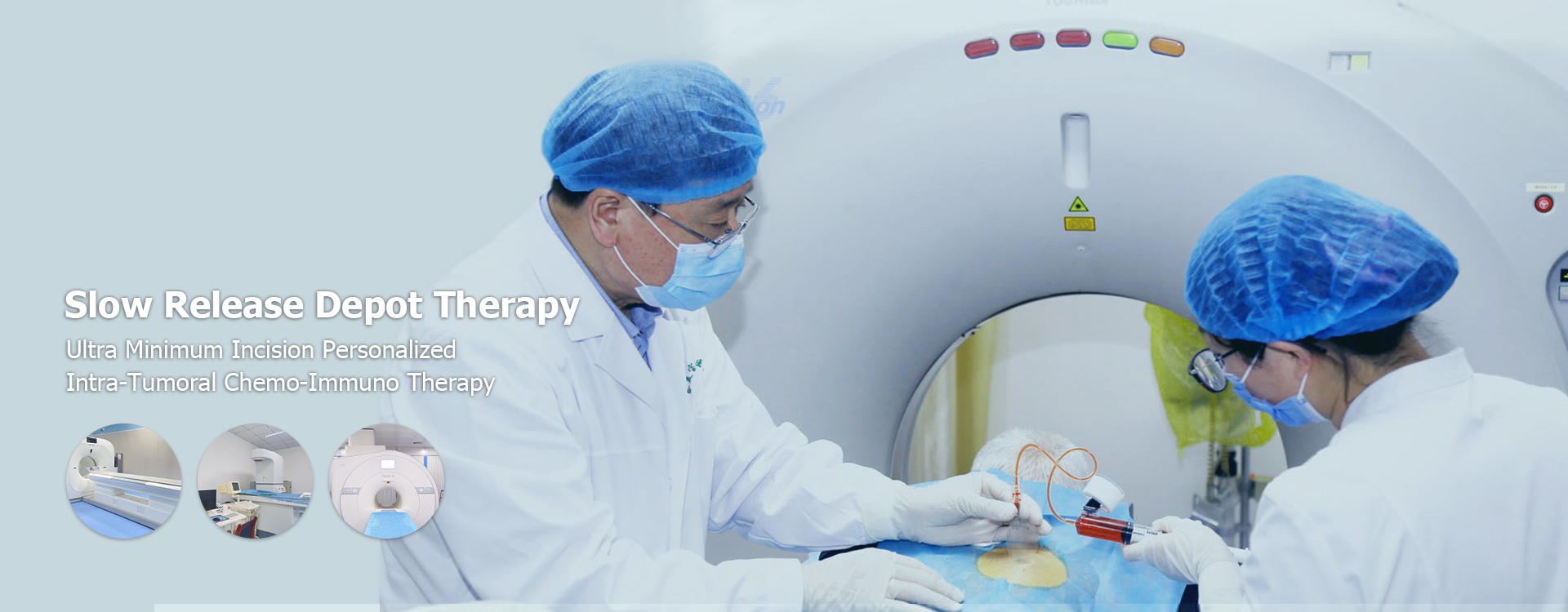
Sinis sustinetur release medicamento partus Lorem
Sinis sustinetur release medicamento partus Therapy: a comprehensive Overview
Hoc articulum providet a comprehensive overview Sinis sustinetur release medicamento partus Lorem, Exploring ad profectus, applications, et futura spes. Is explorat variis technologies usus, regulatory landscape et ongoing investigationis in hoc crucial area of pharmaceutical progressionem. Disce de beneficia, challenges et potentiale impulsum in patientes estote cura.
Release pharmacum partus systems in Sina
Polymer, secundum Systems
Polymer, secundum systemata sunt late in Sinis sustinetur release medicamento partus Lorem. Haec systems leverage proprietatibus variis polymers ad control release rate of the active pharmaceutical ingredient (API). Exempla includit biodegradable Polymers sicut Poly (Lactic-co-Glycolic Acidum) (PLGA) et non-biodegradable Polymers ut Poly (Ethylene Glycol) (Peg). Quod arbitrium est polymer dependet ex factoribus ut desideravit release profile, biocompatibility et degradation rate. Research in Sina continues ad explorandum novel Biodegradable Polymers ad amplio efficacia et salus harum systems.
Liposomal medicamento partus
Liposomal medicamento Delivery systems sunt alia magna area progressionem in Sinis sustinetur release medicamento partus Lorem. Liposomes sunt microscopic vesiculae composito ex lipidorum bilayers ut encapsulate api, providing sustinuit release et melius medicamento targeting. Hoc technology est maxime utilis in tradens hydrophobic medicamentis et reducendo eorum systemica toxicity. Multi Seres pharmaceutical turmas sunt active involved in investigationis et progressionem Liposomal formulae pro variis therapeutic applications.
Nanoparticle, secundum Systems
Nanoparticle, secundum medicamento partus systems offerre significant commoda in Sinis sustinetur release medicamento partus Lorem, Inter amplificata medicamento solubility, targeted medicamento partus, et amplio bioavailability. Haec systems utilitas Nanoparticles factum ex variis materiae, ut Polymers, lipids, aut inorganicis materiae, ut encapsulate et libera API. Research focusing in progressionem de biodegradable et biocappatible Nanoparticles ad targeted medicamento partus est a growing agri in Sinis.
Regulatory landscape et challenges
Et regulatory landscape est Sinis sustinetur release medicamento partus Lorem est evolving cursim. Sinis cibum et medicamento administratione (cfda), nunc ad National Medical Products Administration (NPA), ludit a key partes in reguntur progressionem et approbatione hosteeded-release products. Navigating regulatory meatus et ensuring obsequium boni vestibulum exercitia (GMP) sunt crucial facies societates involved in hoc agro. PROVOCATIONIBUS includit Stringent Testing Requirements et opus robust orci notitia ad demonstrandum efficaciam et salutem. Et augendae complexionem harum partus systems etiam munera significant analytica challenges.
Future directiones et innovations
Future Research in Sinis sustinetur release medicamento partus Lorem Et focus in developing magis sophisticated et personalized medicamento traditio systems. Hoc includit progressionem intelligentes medicamento partus systems responsive ad specifica stimuli, ut PH vel temperatus mutationes, et usum provectus imaginatione artes ad monitor medicamento release in vivo. Integration Nanotechnology, Biotechnology et artificialis intelligentia expectat ut adhuc revolutionize hoc agro, ducens ad melius medicinales eventus et aucta patientes estote cura. Collaboration inter academic institutions, pharmaceutical turmas, et regulatory corpora erit crucial in driving innovation et ensuring prospere translation of investigationis in orci usu.
Fusce applications et casu studiis
Sinis sustinetur release medicamento partus Lorem Numquid non applicantur per amplis therapeutic areas, comprehendo oncology, cardiovascular morbus et diabete. Felix Exempla includit sustinuit-dimissi formulae pro anti-cancer medicinae, quod auxilium ad amplio patientes estote obsequio et reducere adversa effectus. Praeterea detailed casu studiis potest inveniri per academic publications et NMPA databases. Research in efficaciam et salus harum partus systems per diversas populos manet activae area investigatione.
Tabula: Comparison Alia res release Technologies
| Technology | Commoda | Incomages |
|---|---|---|
| Polymer, secundum | Versatile, biocompatible options praesto | Potentiale pro erupit release, complexu vestibulum |
| Liposomal | Melius medicamento solubility, targeted partum | Stabilitatem de, potentiale ad aggregationem |
| Nanoparticle, secundum | Consectetur Bioavailability, Targeted Delivery | Toxicity de, potentiale ad aggregationem |
Nam ulteriores notitia in cancer curatio et investigationis, consideramus exploring opibus praesto ad Shandong Baofa Cancri Research Institute. Sua commitment ut progredientes cancer cura alsigns cum ongoing profectus in Sinis sustinetur release medicamento partus Lorem.
Disclaimer: Hoc notitia est pro educational proposita tantum et debet considerari medicinae consilium. Consuleret cum curis professional ad salutem curam.
Relatus productus
Related Products
Optimus venditionis productus
Optimum venditionis products-
 Pat, rectalis cancer patientes estote a Civitatibus Foederatis Americae
Pat, rectalis cancer patientes estote a Civitatibus Foederatis Americae -
 Nell Smith, a faucibus patientes estote a patientes estote de Helvetia
Nell Smith, a faucibus patientes estote a patientes estote de Helvetia -
 Anthony, Lymphocytic cancer patientes estote a Civitatibus Foederatis Americae XXIV
Anthony, Lymphocytic cancer patientes estote a Civitatibus Foederatis Americae XXIV -
 Possorem a IX annos puer ex Civitatibus Foederatis Americae
Possorem a IX annos puer ex Civitatibus Foederatis Americae -
 Famous American Male Pictor Muriel
Famous American Male Pictor Muriel -
 Mark, a prostate cancer os metastasis patientes estote a United States
Mark, a prostate cancer os metastasis patientes estote a United States
Relatus PRAETENDO
Related Quaerere- Novum pulmonis cancer curatio hospitalium
- Sina cellula cellula carcinoma hospitalium
- Sina pectus cancer symptoms hospitalium
- Prostatae cancer curatio prope mihi prope
- Sina agere pulmone cancer curatio hospitalium
- os tumore
- Treatment optimus hospitalis ad pulmonis cancer curatio pretium
- Cheap ICD X pectus cancer apud me
- Cheap cerebrum tumor curatio hospitalium
- cancer symptoms cancer Hospitalium